|
CDISC Transformer
Development of integrated clinical trials data repository and
CDISC transformer module |
|
|
|
|
|
|
- Escalating interests of clinical trials and multi-national and multi-institutional clinical trials are increasing.
- ClinicalTrials.gov trial registry contain and open information on more than 100,000 clinical studies,
and even provides summary result data. It aims to improve the clinical resesarch enterprise.
- However, clinical trial enterprise in domestic is difficult to be advanced due to heterogeneous data formats and controlled vocabularies
- Based on the international standard Clinical Data Interchange Standards Consortium (CDISC),
we develop clinical trial data transforming modules and model to integrate clinical trial information.
|
| ● STRUCURE OF CDISC TRANSFORMER
(top) |
|
|
|
|
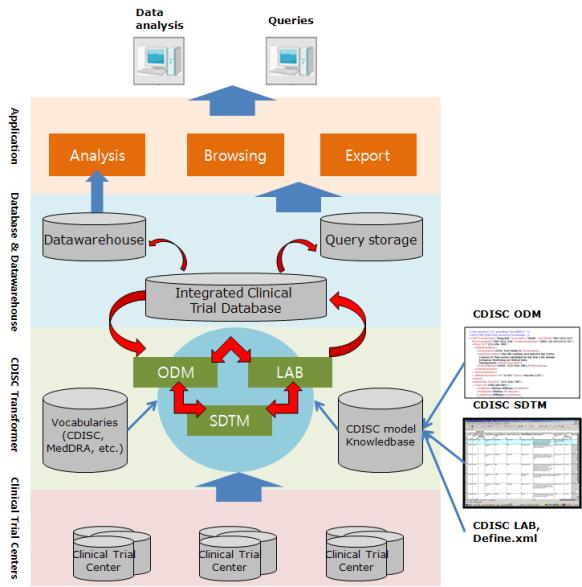 |
| |
1 Part : Development of integrated clinical information registry and integrated analysis system
- Analysis of standard model for clinical trial information
- Design of databases and datawarehouse for managing and integrating clinical trial information
- Development of databases and datawarehouse
- Development of statistical analysis system and mining tool
2 Part : Development of CDISC transformer base on CDISC standard model for clincal trial data interoperability
- Understand CDISC standard models
- Research controlled vocabulary used in CDISC model
- Design and development of the mapping rules for interacting among clinical trial data and standard model structure
- Mapping clinical trial data into controlled vocabulary
- Development of CDISC Transformer system based on mapping rule
- Development of clinical trial data transmitting protocol based on standard formet
|
| ● RESEARCH GOAL AT EACH YEAR
(top) |
|
|
|
|
| |
| Year |
Goal |
Research Content |
| 1st Year |
- Understand and analysis of standard model for clinical trial information
- Design of databases and datawarehouse for managing and integrating clinical trial information
- Research controlled vocabularies used in CDISC model
- Development of CDISC Transformer system based on mapping rule
|
- Research of CDISC models (ODM, SDTM, ADaM, LAB and etc.)
- Research the status of clinical trial enterprise and how CDISC models are utilized in international/domestic
- Understand clinical trial process and general term used
- Design of databases to represent and cover entire CDISC models and relative controlled terminologies
- Desgin of a datawarehouse for analysis on clincl trial information
|
| 2nd Year |
- Development of databases and datawarehouse for managing and integrating clinical trial information
- Development of statistical analysis system and mining tool
- Mapping clinical trial data with controlled vocabulary
- Development of CDISC Transformer system based on mapping rule
- Development of clinical trial data transmitting protocol based on standard formet
|
- Development of databases and datawarehouse for managing and integrating clinical trial information
- Development of statistical analysis system based on CDISC ADaM model (using ClinicalTrials.gov summary result data)
- Mapping clinical trial data with controlled vocabularies (UMLS)
- Development of CDISC Transformer system (compliant with CDISC SDTM and ODM models)
- Development of clinical trial data transmitting protocol based on standard formet
|
|
| |
|
|
| |
| Year |
Result |
Publication |
| 1st Year |
|
- CDISC Transformer: a metadata-based transformation tool for clinical trial and research data into CDISC standards. Yu Rang Park, Hye Hyeon Kim, Hwa Jeong Seo, Ju Han Kim* KSII Transactions on Internet and Information Systems 2011;5; 1830-184
|
| 2nd Year |
- CDISC Transformer: meta-data driven semi-automatic transformation of CDISC ODM standard model to CDISC SDTM standard model
- Understand clinical trial analysis data as converting ClinicalTrials.gov summary result data to CDISC ADaM standard model
|
|
|
